Adsorption
Shale reservoirs have immense capacity for methane storage. The mechanism by which this occurs is called adsorption. In adsorption, molecules of gas become attached to the surface of organic material in shale. Nearly all the gas stored by adsorption exists in a condensed, near-liquid state. Adsorption can be visualized by imagining a magnet attached to a metal surface, or lint attached to a sweater. This is different from absorption where one substance becomes trapped inside another, such as a sponge soaking up water. Adsorption is a reversible process, because it involves weak attraction forces.
Typically, shale reservoirs can store far more gas in the adsorbed state than conventional reservoirs can hold by compression at pressures below 1000 psia. Since the volume of a cleat or fracture system is small when compared to the volume of the reservoir, free gas only accounts for a small portion of the gas stored in shale. As a result, the pressure volume relationship is often described by the desorption isotherm only.
Langmuir isotherm formulation
The release of adsorbed gas is commonly described by a pressure relationship called the Langmuir isotherm. The Langmuir adsorption isotherm assumes that the gas attaches to the surface of the shale, and covers the surface as a single layer of gas (a monolayer). At low pressures, this dense state allows greater volumes to be stored by sorption than is possible by compression.
The typical formulation of the Langmuir isotherm is:
where Cg is the gas content measured in scf / ton of shale.
Langmuir volume
The Langmuir volume is the maximum amount of gas that can be adsorbed at infinite pressure. The following plot of a Langmuir isotherm demonstrates that gas content asymptotically approaches the Langmuir volume as pressure increases to infinity.
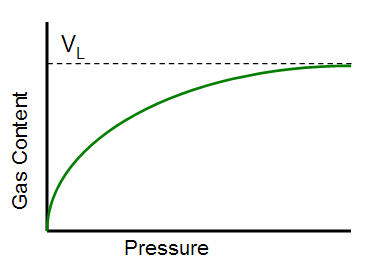
The units for Langmuir volume are scf / ton (volume gas per mass of shale). This can be converted to scf/ft3 (volume of gas per volume of shale) by multiplying it by the bulk density.
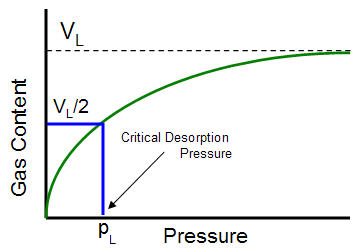
Langmuir pressure
The Langmuir pressure, or critical desorption pressure, is the pressure at which one half of the Langmuir volume can be adsorbed. As seen in the figure below, it changes the curvature of the line and thus affects the shape of the isotherm.
Isotherm variability
An isotherm is based on the assumption that the temperature is constant. The adsorption capacity decreases as temperature increases. When doing any analysis on shale, it is important to use the isotherm based on the reservoir temperature.