Capillary pressure (Pc) is the pressure difference across the interface between two immiscible fluids arising from capillary forces. These capillary forces are surface tension and interfacial tension. In porous media, the capillary pressure is the difference between the pressure in the non-wetting phase and the pressure in the wetting phase. It is defined by:
In oil-water systems, water is typically the wetting phase; while for gas-oil systems, oil is typically the wetting phase.
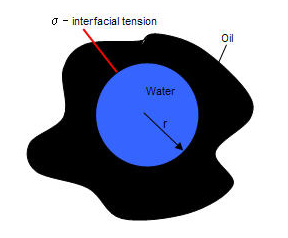
In a drop of water in oil, the interfacial tension is denoted by σ and has the following capillary pressure:
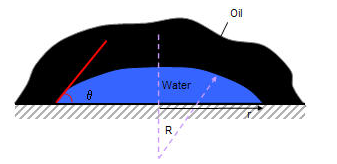
A drop of water on a surface with contact angle θ (where θ < 90° is water wet) has the following capillary pressure:
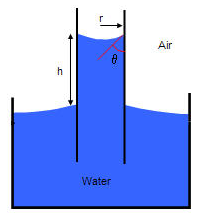
Water in a capillary tube with contact angle θ, where h is the height the water rises, has the following capillary pressure:
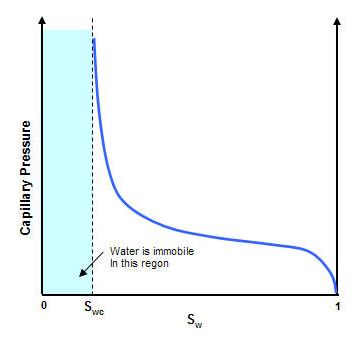
The shape of the curve depends on pore sizes, size distribution, and fluid properties. The permeability of the reservoir rock can alter the capillary pressure curve. If the value of the permeability is lower, the pore size is smaller, and the capillary pressure is higher.