Gas
A gas can be defined as a homogenous fluid of low density and low viscosity, which has neither independent shape nor volume. It expands to completely fill the vessel in which it is contained. The properties of gases differ from liquids mainly because the molecules in gases are much further apart than liquids. The ideal gas law states:
This equation has limited practical value because no known gas behaves as an ideal gas. However, this equation does describe the behavior of most real gases at low pressures, and it serves as a starting point to develop equations of states for real gases at elevated pressures. Furthermore, the behaviors of most real gases do not deviate drastically from the behavior predicted by this equation. By inserting a correction factor (Z) into the ideal gas equation, real gas behavior can be accurately predicted.
The correction factor is called the gas compressibility factor; and represents the deviation from ideal gas behavior.
Gravity
Gas gravity is the molar mass (molecular weight) of the natural gas divided by the molar mass of air (28.94 kg / kmol). It ranges from 0.55 for dry sweet gas to approximately 1.5 for wet sour gas. Petroleum gases typically have a gravity of about 0.65.
The gas gravity affects the calculations of gas viscosity, compressibility, compressibility factor, and solution gas-oil-ratio.
Compressibility factor
The gas compressibility factor of a natural gas is a measure of its deviation from ideal gas behavior. The gas compressibility factor is the ratio of the volume actually occupied by a gas at a given pressure and temperature to the volume the gas would occupy at the same pressure and temperature, if it behaved like an ideal gas.
The gas compressibility factor is usually between 0.8 and 1.2, but it can be as low as 0.3 and as high as 2.0. It is used in the calculation of gas pseudo-pressures, and in converting gas volumes and rates from standard conditions to reservoir conditions (and vice versa). It is sometimes called the super-compressibility factor, and is often confused with the term "compressibility", which is the change in volume per unit change in pressure. The gas compressibility factor directly affects the gas compressibility.
Compressibility
Gas compressibility is the relative change in volume per unit change in pressure. The gas compressibility should not be confused with the gas compressibility factor. The gas compressibility is a very strong function of pressure and increases as the pressure decreases.
Gas compressibility, when plotted versus pressure, shows a discontinuity at the dew point pressure. When the gas is undersaturated (that is, the pressure is greater than the dew point pressure), mathematically it can be expressed as:
or
Where p is the specified pressure, Bgd is the dry gas formation volume factor, and Z is the gas compressibility factor at that pressure. Thus, the magnitude of gas compressibility is of the order of 1/p.
When the gas is saturated (that is, pressure is less than the dew point pressure), it is expressed as:
Where Bo is the oil formation volume factor, Rs is the solution gas ratio, and Rv is the vaporized oil ratio.
Formation volume factor
The gas formation volume factor is the gas volume at reservoir conditions divided by gas volume at standard conditions. It is used to convert surface measured volumes to reservoir conditions, assuming that the fluid is in a single-gas phase despite temperature and pressure changes. Defined below, it is a function of the fluid composition and the pressure / temperature ratio between reservoir (in-situ) and standard conditions (14.65 psia and 519.67 °R or 60 °F):
It is a very strong function of pressure, and a weak function of temperature and gas composition.
The dry gas formation volume factor is the gas volume at reservoir conditions divided by the gas volume at standard conditions. It is used to describe the gas volume change from the reservoir to the surface, taking into account the phase change between gas and liquid.
Vaporized oil ratio (Rv)
For condensate gas and volatile oil reservoirs with a gas cap, the liquid component in the reservoir gas needs to be considered. This is described by the vaporized oil rRatio (Rv). Rv is defined as the ratio of the volume of produced stock tank condensate or oil to the volume of produced separator gas (commonly used unit is stb/MMscf). It is used in the modified black oil (MBO) models.
Viscosity
Gas viscosity is a measure of the resistance to flow exerted by the gas and is given in units of centipoises (cp). Higher values indicate more resistance to flow. For gas, the viscosity increases with increasing temperature and pressure. As pressure decreases, gas viscosity decreases. The molecules are simply further apart at lower pressure and move past each other more easily.
Experimental determination of gas viscosity is difficult. Usually it is not measured but obtained from correlations, which include corrections for H2S, CO2, and N2. Gas viscosity is used in numerous equations, most notably in the definitions of pseudo-pressure and pseudo-time. Typically, gas viscosity is in the range of 0.015 to 0.03 cp or 15 to 30 micro-Pa-s.
Critical temperature
Gases can be converted to liquids by compressing the gas at a suitable temperature. As the temperature increases, the kinetic energies of the particles that make up the gas also increase, and the gases become more difficult to liquefy. The critical temperature of a substance is the temperature at and above which vapor of the substance cannot be liquefied, no matter how much pressure is applied. For example, the critical temperature of water is 374°C and carbon dioxide is 31.2°C.
Critical temperature represents the temperature above which distinct liquid and gas phases do not exist. As the critical temperature is approached, the properties of the gas and liquid phases become the same, resulting in one phase known as supercritical fluid. The critical temperature value is used in the definition of reduced temperature (Tr = T / Tc), which in turn is used directly in correlations or equations of state to determine various PVT properties of natural gases (for example, viscosity, compressibility, gas compressibility factor, etc.).
Critical pressure
The critical pressure of a substance is the pressure required to liquefy a gas at its critical temperature. For example, the critical pressure of water is 217.7 atm and carbon dioxide is 73.0 atm.
Critical pressure represents the pressure above which distinct liquid and gas phases do not exist. As the critical pressure is approached, the properties of the gas and liquid phases become the same, resulting in one phase known as supercritical fluid. The critical pressure value is used in the definition of reduced pressure (pr = p / pc), which in turn is used directly in correlations or equations of state to determine various PVT properties of natural gases (for example, viscosity, compressibility, gas compressibility factor, etc.).
Oil
Correlations of physical properties of reservoir crude oils are more complicated than those for natural gas because of the many different components they contain. Although most components are hydrocarbons, the larger molecule components can be different chemical classes. These larger, heavier components can strongly influence the behavior of the mixture. Also the mixing rules for liquids are considerably different than those for gases because of the complex nature of hydrocarbon liquids.
There are three main sources for developing key oil properties.
1. Subsurface sampling of the produced fluid at reservoir conditions. This is the best method because the complex mixtures of hydrocarbons make each oil unique. The individual properties can then be determined empirically in a laboratory.
2. Surface sampling at a separator where the rate of flow for each fluid, gas and liquids, is measured along with their respective compositions. These fluids are then recombined in the laboratory at reservoir conditions, and the resulting fluid is used to empirically determine key oil properties.
3. Correlations are often used when only key parameters, such as the density of the produced oil and the volume of solution-gas evolved, are known. Correlations should be used only after being proved / tuned with laboratory measurements for subsurface samples of analogous oils.
Gravity
Oil gravity relates the density of oil to that of the density of water. The oil gravity has a very strong effect on the calculated oil viscosity and solution gas-oil ratio. It has an indirect effect on the oil compressibility and the oil formation volume factor because these variables are affected by the solution gas-oil ratio.
The American Petroleum Institute (API) developed a specific gravity scale that measures the relative density of various petroleum liquids. API gravity is gradated in degrees on a hydrometer instrument and was designed so that most values would fall between 10° and 70° API.
Usually the oil gravity is readily known. It ranges from 45 °API (light oil) through 20 °API (medium density) to 10 °API (heavy oil). The conversion from API gravity (oil field units) to relative gravity (relative to water) is:
The conversion of oil relative gravity to oil density is:
where:
ρw ≈ 62.37 lb∙m / ft3 or 1000 kg / m3
Formation volume factor
The oil formation volume factor (FVF) is defined as the ratio of the volume of oil and dissolved gas at reservoir (in-situ) conditions to the volume of oil at stock tank (surface) conditions. Since most measurements of oil and gas production are made at the surface, and the fluid flow takes place in the formation, volume factors are needed to convert measured surface volumes to reservoir conditions. It is defined as:
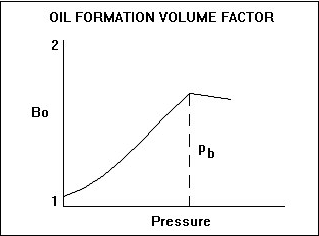
The oil formation volume factor is influenced by two main factors: solution gas and the compressiblity of oil. The dominant factor is solution gas. As pressure increases, the amount of solution gas that the oil can dissolve increases such that the oil swells, and so the formation volume factor exceeds 1.0. After there is no remaining free gas available to dissolve in the oil, further increases in pressure result in a decline in formation volume factor due to the second influencing factor – the compressibility of oil. As shown in the diagram below, the oil formation volume factor is dominated by swelling below the bubble point pressure (due to dissolved gas), and by compressibility above the bubble point pressure (because all available gas is now dissolved).
Shrinkage
Shrinkage is the inverse of the formation volume factor for oil, and represents the difference between the volume of oil in the reservoir and its volume when produced to the surface (standard pressure and temperature). The value of shrinkage is generally between 0.5 and 1, and the change in volume is due to solution gas coming out of the oil as the pressure decreases.
Compressibility
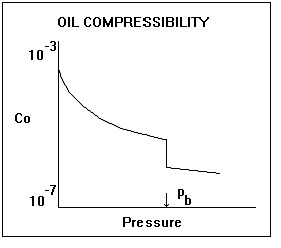
Oil compressibility is defined as the relative change in oil volume per unit change in pressure. Oil compressibility is a source of energy for fluid flow in a reservoir. In an undersaturated reservoir, it is a dominant drive mechanism, but for a saturated reservoir, it is overshadowed by gas compressibility effects due to the evolution of dissolved gas. Oil compressibility is a component of total compressibility, which is used in the determination of skin, dimensionless time, and material balance.
Oil compressibility, when plotted versus pressure, shows a significant discontinuity at the bubble point pressure. Above this pressure (undersaturated condition), the oil is a single-phase liquid consisting of oil and dissolved gas. The compressibility of this liquid can be measured in the laboratory, and it is a weak function of pressure. Mathematically, it can be expressed as:
Where Bo is oil formation volume factor.
Below the bubble point pressure (saturated condition), gas comes out of solution causing a sharp increase in compressibility, which causes the discontinuity shown in the plot. While below the bubble point pressure, oil compressibility becomes a much stronger function of pressure. It can be expressed as:
Where Bgd is the dry gas formation volume factor, Rs is the solution gas ratio, and Rv is the vaporized oil ratio.
Solution gas-oil ratio
The solution gas-oil ratio is the amount of gas dissolved in the oil at any pressure. It increases approximately linearly with pressure and is a function of the oil and gas composition. A heavy oil contains less dissolved gas than a light oil. In general, the solution gas-oil ratio varies from 0 (dead oil) to approximately 2000 scf/bbl (very light oil). The solution gas-oil ratio increases with pressure until the bubble point pressure is reached, after which it is a constant, and the oil is said to be undersaturated.
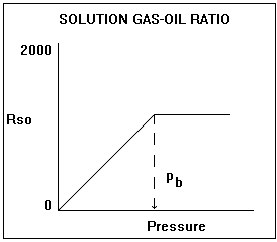
The solution gas-oil ratio is a significant component of the PVT correlations. It has a very significant influence on the oil formation volume factor, the oil viscosity, and the oil compressibility.
Viscosity
Oil viscosity is a measure of the resistance to flow exerted by the oil, and is given in units of centipoises (cP). Higher values indicate greater resistance to flow. For oil, the viscosity decreases with increasing temperature and pressure (up to the bubble point). Above the bubble point pressure, oil viscosity increases minimally with increasing pressure as shown below. It is a very strong function of reservoir temperature, oil gravity, and the solution gas-oil ratio.
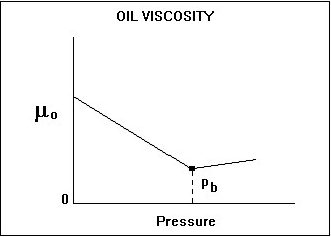
Oil viscosity is measured as a function of pressure in most PVT laboratory measurements. Occasionally, a routine oil analysis report quotes the oil viscosity (and the kinematic viscosity). These measurements are at stock tank conditions and should not be used as the in-situ oil viscosity. Dead-oil viscosity is defined as the viscosity of crude oil at atmospheric pressure (no gas in solution) and system temperature. There are several correlations available for estimating oil viscosity at reservoir conditions, but great care must be taken since they are very sensitive to the oil gravity and solution gas-oil ratio inputs. The oil viscosity at reservoir conditions can vary from 10,000 cP for a heavy oil to less than 1 cP for a light oil.
Bubble point pressure
The bubble point pressure is defined as the pressure at which the first bubble of gas comes out of solution. At this point, we can say the oil is saturated – it cannot hold anymore gas. Above this pressure, the oil is undersaturated, and the oil acts as a single-phase liquid. At and below this pressure, the oil is saturated, and any lowering of the pressure causes gas to be liberated resulting in two-phase flow.
Water
Estimating reservoir water properties is important for reservoir engineering calculations, specifically for those with water influx. Also, water is often a very important liquid component of an oil and gas production system. The physical properties of water play an important role in multiphase flow calculations.
Because water composition is only generally affected by dissolved solids, correlating water properties is relatively simple. Also, changes in the physical properties of water as a function of temperature and pressure are relatively small and usually can be predicted.
Water-specific gravity
Water-specific gravity is defined as the density of the water divided by the density of water at standard conditions (62.3 lb / ft3). Water contained in a reservoir is saline and usually has a specific gravity greater than 1.0. Water-specific gravity has no effect on calculated properties such as water compressibility, formation volume factor, and viscosity. It is used however, in the wellbore pressure-drop calculations when converting pressures from wellhead to sandface.
Salinity
Salinity is the quantity of dissolved salt in water, and is usually expressed as the mass of dissolved salt in a unit volume solution, in mg / L, or parts per million (ppm). Salinity is often reported as total dissolved solids (TDS).
Viscosity
Water viscosity is a measure of the resistance to flow exerted by water. Higher values indicate more resistance to flow. For water, the viscosity decreases with increasing temperature, and increases with increasing pressure. Water viscosity is a very weak function of pressure. Water at room temperature is approximately equal to 1 cP. In a reservoir, it is typically between 0.5 to 1 cP. This is due to the higher temperature, salinity, and the solution-gas content of water.
Compressibility
The compressibility of any substance is the change in volume per unit volume per unit change in pressure. Water compressibility is a source of energy for fluid flow in a reservoir, but it is only significant when there is no free gas present in the reservoir. The value of water compressibility can be obtained from laboratory PVT measurements, or it can be determined from correlations. The magnitude is approximately between 1.0 x 10-6 and 9.0 x 10-6psi-1. It is a weak function of pressure, temperature, and salinity.
Formation volume factor
The water formation volume factor is defined as the ratio of the volume of water at reservoir (in-situ) conditions to that at stock tank (surface) conditions. This factor is used to convert the flow rate of water (at stock tank conditions) to reservoir conditions.
The water formation volume factor can be measured in the laboratory, or it can be determined from correlations. In most situations, the water formation volume factor is very close to one, and so most practitioners tend to set it to one. It is a very weak function of pressure, temperature, and salinity.
Dissolved gas / water ratio
The solution gas / water ratio is the amount of gas dissolved in the water. It increases approximately linearly with pressure, and is a function of the water and gas composition. Quantitatively, the solubility of gas in water is considerably less than that of gas in oil.